electronic configuration ag|ground state electron configuration ag : Tagatay Mar 23, 2023 30 puta dnevno, 20 igrača dobija bonus u vredno; Saznaj više. Slot. DOŽIVI EUROFORIJU NA SLOTOVIMA. 🚨 Došli smo do kraja EUROSLOTA i ostalo je da izvučemo dobitnika velikog džekpota, čija je vrednost došla do 2.550.000 RSD, kao i dobitnike deset džekpotova u vrednosti od po 100.000 RSD. Izvlačenje dobitnika će biti u nedelju, 14.
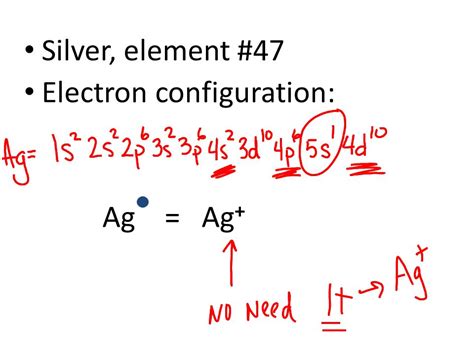
electronic configuration ag,The ground-state electron configuration of silver is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s1. This electron configuration shows that the last shell of silver has an electron and the d-orbital has a total of ten electrons. Therefore, the valence electronsof silver are one. The elements that form . Tingnan ang higit paThe total number of electrons in silver is forty-seven. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in silver in . Tingnan ang higit paground state electron configuration agScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electronsof . Tingnan ang higit pa
Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the nucleus is called the orbital. The sub-energy . Tingnan ang higit pa
Mar 23, 2023 electronic configuration ag The electron configuration for silver (Ag) is based upon the place meant of silver in the fifth row of the periodic table in the 11th column of the periodic table or the .
640. 79K views 3 years ago. To write the configuration for the Silver and the Silver ion, first we need to write the electron configuration for just Silver (Ag). We first .electronic configuration ag ground state electron configuration agg. ? Solution. Verified by Toppr. The electron configuration for silver (Ag) is based upon the place meant of silver in the fifth row of the periodic table in the 11th column of the .
What is the electron configuration and orbital diagram of: Na + P 3– Al 2+ Fe 2+ Sm 3+ Solution. First, write out the electron configuration for each parent atom. We have chosen to show the full, unabbreviated . Electronic configuration of Ag is 1s2 2s22p6 3s2 3p6 3d10 4s2 4p6 4d10 5s1. Silver is the transition metal atom its symbol is Ag. It is the 47th periodic table .
Write the Electron Configuration of Silver (Ag and Ag+) | Channels for Pearson+. with Jules. Get help from an AI Tutor. Ask a question to get started. Next video. General .The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons .Silver electron configuration. ← Electronic configurations of elements. Ag (Silver) is an element with position number 47 in the periodic table. Located in the V period. Melting .
Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the . Solution. 1. Locate the atom on the periodic table. 2. Locate the noble gas element in the period above the element of interest. 3. Continue the electron configuration from the noble gas until you reach the element of interest. 4. Put the noble gas in brackets and write the remainder of the electron configuration.

Hund's Rule. Hund's rule suggests that electrons prefer parallel spins in separate orbitals of subshells. This rule guides us in assigning electrons to different states in each sub-shell of the atomic .
Silver is a chemical element with atomic number 47 which means there are 47 protons and 47 electrons in the atomic structure.The chemical symbol for Silver is Ag. Electron Configuration and Oxidation States of Silver. Electron configuration of Silver is [Kr] 4d10 5s1. Possible oxidation states are +1. Electron Configuration
There are several important exceptions to the general pattern for electron configurations of the elements. In this video we will look four different exceptio.Electron Configuration -The Electron Configuration of an Element Describes how Electrons are Distributed in their Atomic Orbitals. In Electronic Configuration electrons are arranged in various shells, Subshell and Orbital by following certain rules. To Learn how to Write Electronic Configurations, Detailed Explanation, Filling of orbital with FAQs, Visit .The electronic configuration of each element is decided by the Aufbau principle which states that the electrons fill orbitals in order of increasing energy levels. The equation is: 1s<2s<2p<3s<3p<4s<3d<4p<5s<4d<5p<6s<4f<5d<6p<7s<5f<6d<7p. The concept of electronic configuration has replaced the older concept of valency and valence electrons. Referring to either Figure 6.4.3 6.4. 3 or 6.4.4 6.4. 4, we would expect to find the electron in the 1 s orbital. By convention, the ms = +1 2 m s = + 1 2 value is usually filled first. The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2.Silver is a chemical element of the periodic table with chemical symbol Ag and atomic number 47 with an atomic weight of 107.868 u and is classed as a transition metal. . Electron configuration chart. 1s 2: 2s 2: 2p 6: 3s 2: 3p 6: 3d 10: 4s 2: 4p 6: 4d 10: 5s 1: Electrons per shell: 2, 8, 18, 18, 1: Valence electrons : 1: Valency electrons : 1:
The first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2 s orbital (Figure 5.1.3 or 5.1.4 ). Thus, the electron configuration and orbital diagram of lithium are:
Ag (Silver) is an element with position number 47 in the periodic table. Located in the V period. Melting point: 961.9 ℃. Density: 10.49 g/cm 3 . The order of filling the orbitals with electrons in the Ag atom is an exception to the rule. Expected electronic configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d9 But in reality, one electron .
Electron configurations are written using the principal quantum number n, followed by the orbital (s, p, d, or f) with the total number of electrons written as a superscript. Example: 1s 2 For writing ground state electron configurations, a few main steps should be followed. Find the amount of electrons in the atom. Example: Na: 11 e . So if you're thinking about the subshell, the s subshell could fit two electrons, the p subshell can fit six electrons, the d subshell can fit 10 electrons, and the f subshell can fit 14 .
Introduction. The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. In doing so, we obtain three quantum numbers (n,l,m l), which are the same as the ones obtained from . The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number . Check me out: http://www.chemistnate.com An atom's electron configuration describes the way its electrons fill sublevels when the atom is in its ground state. Atoms seek the most stable electron configuration, so sublevels are half-filled or fully-filled .
La configuration électronique des éléments périodiques montre le nombre total d'électrons disposés dans leur orbitale atomique. Voyons la configuration électronique de Ag. La configuration électronique de Ag est 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 1. L'argent est l'atome de métal de transition dont le symbole est Ag.
electronic configuration ag|ground state electron configuration ag
PH0 · valency of silver
PH1 · silver ion electron configuration
PH2 · pt electronic configuration
PH3 · ground state electron configuration ag
PH4 · electron configuration silver
PH5 · cu electronic configuration
PH6 · atomic configuration of silver
PH7 · Iba pa